Home Sectors Life Sciences CRO/Pharma
Translation for Life Sciences : our experience with the Pharmaceutical and CRO world
Ubiqus is a leading global language service provider. Since our foundation in 1991, we have gained significant experience in the field of Life Sciences, particularly in the medical, pharmaceutical and CRO world. Working with medium-sized companies to large multinational groups, we deliver scientific and non-scientific projects in over 100 language combinations.
We provide high-quality, regulatory-compliant translations, on time and at a cost-effective price.
Translation solutions for the Pharmaceutical and CRO world
High-Quality Translations
Regulatory-Compliant Translations
On-Time Deliveries
High-Quality Translations
We have built a network of more than 2,000 translators, reviewers and subject matter experts. Each of which has been recruited, selected and approved by our Quality Control department.
All our language professionals are in-country native speakers. To be selected to work on a pharmaceutical or CRO project, translators must have:
- a strong medical, pharmaceutical, scientific or clinical background in the specific therapeutic area,
- experience working with the specific type of document
and they must demonstrate that they are up to date with the relevant regulations.
All translations are delivered with a Certificate of Translation (CoT).
Regulatory-Compliant Translations
We hold ISO 9001:2015 and ISO 17100:2015 certifications at Ubiqus Group level. In addition, our Quality Control department provides regular training to ensure that all those involved in a project are kept up to date on any related regulatory changes.
On-Time Deliveries
A dedicated Project Management team works with you to define solutions and procedures that meet your translation needs and to manage all your projects to ensure they are delivered on time.
Our extensive network of language professionals enables us to quickly react to urgent translation requests and manage translation peaks. In parallel, our ongoing recruitment programme means that we can adapt our network to market trends and recruit new translators for new or more frequently needed language combinations
In addition, our internal employees and network of professionals are trained to adapt to changes in the content or delivery date during an ongoing project.
OVERVIEW OF OUR CLIENTS IN LIFE SCIENCES
More than 48 million words translated annually related to Life Sciences
More than 13,000 projects
More than 100 language combinations
Involving a network of more than 2,000 language professionals
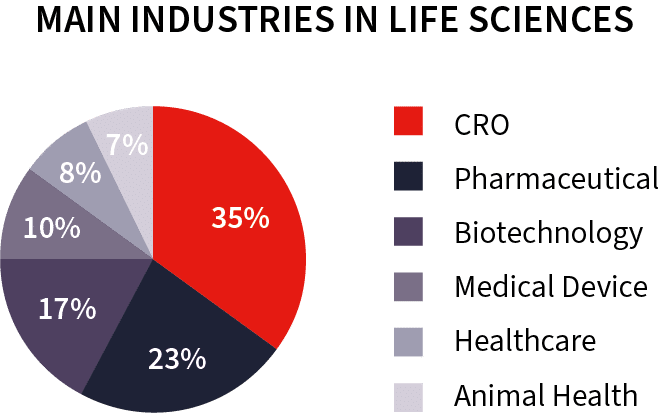
CRO translations: a very unique expertise in Life Sciences
CLINICAL TRIALS
Clinical trials are increasingly conducted on an international basis. There are many advantages to international trials, but they also generate additional challenges for the CRO.
You have to get your documents ready on-time for submission in each country, accurate in all languages, stored in a secure environment, with clear tracking and visibility of the work in progress.
Ubiqus can help you navigate these steps smoothly.
We provide all the services you need throughout the lifecycle of your study: from early stage to post-marketing phase. Furthermore, we can accommodate your specific requirements:
- Target audiences: patient and professional content
- Countries involved: extensive coverage of a range of language combinations
- Therapeutic areas: experts in a range of therapeutic areas
- Content types: Informed Consent Form (ICF), Protocol, Protocol Synopsis, Investigator Brochure, Patient Diary, Questionnaires, Regulatory Affairs and Ethics Committee Correspondence, Contracts, Serious Adverse Event (SAE) and more
- Text adaptation: reworking medical texts for a lay audience
- Formats: all editable formats, PDFs and handwritten documents
Our internal standard operating procedures combined with the latest technology ensure successful outcomes: your translation is in good hands.
REGULATORY AFFAIRS
We understand the importance of patient safety. For this reason, we ensure we have the regulatory and industry-specific knowledge to translate all your regulatory documents accurately and in compliance with regulatory agencies (FDA, EMA, …).
We regularly translate Product Information Leaflets (PILs), Labels, Packaging, Summaries of Product Characteristics (SPCs) and Regulatory Correspondence.
To meet the local regulatory standards, we comply with Quality Review Documents (QRD) template guidelines, as published by the EMA.
We also comply with the Medical Dictionary for Regulatory Activities (MedDRA) and Good Clinical Practice (GCP) guidelines, as published by the ICH.
We use the latest technology to ensure that the client-specific style guides, templates and glossaries we create maximise quality, consistency and compliance.

PHARMACOVIGILANCE
We provide a rapid and reliable service for the translation of Serious Adverse Event (SAE) and Suspected Unexpected Serious Adverse Reaction (SUSAR) reports.
THERAPEUTIC AREAS
MORE SERVICES
- Translation of e-Learning modules
- Marketing translations
- Interpretation services
- Machine translation
- Post-editing
- Linguistic validation
- Transcription
- Sworn translations
- Subtitling/Audiovisual
Technological expertise to increase efficiency
Automatic Speech Recognition (ASR)
Neural Machine Translation (NMT)
Certifications





Shall we talk about your project?
